Key moments
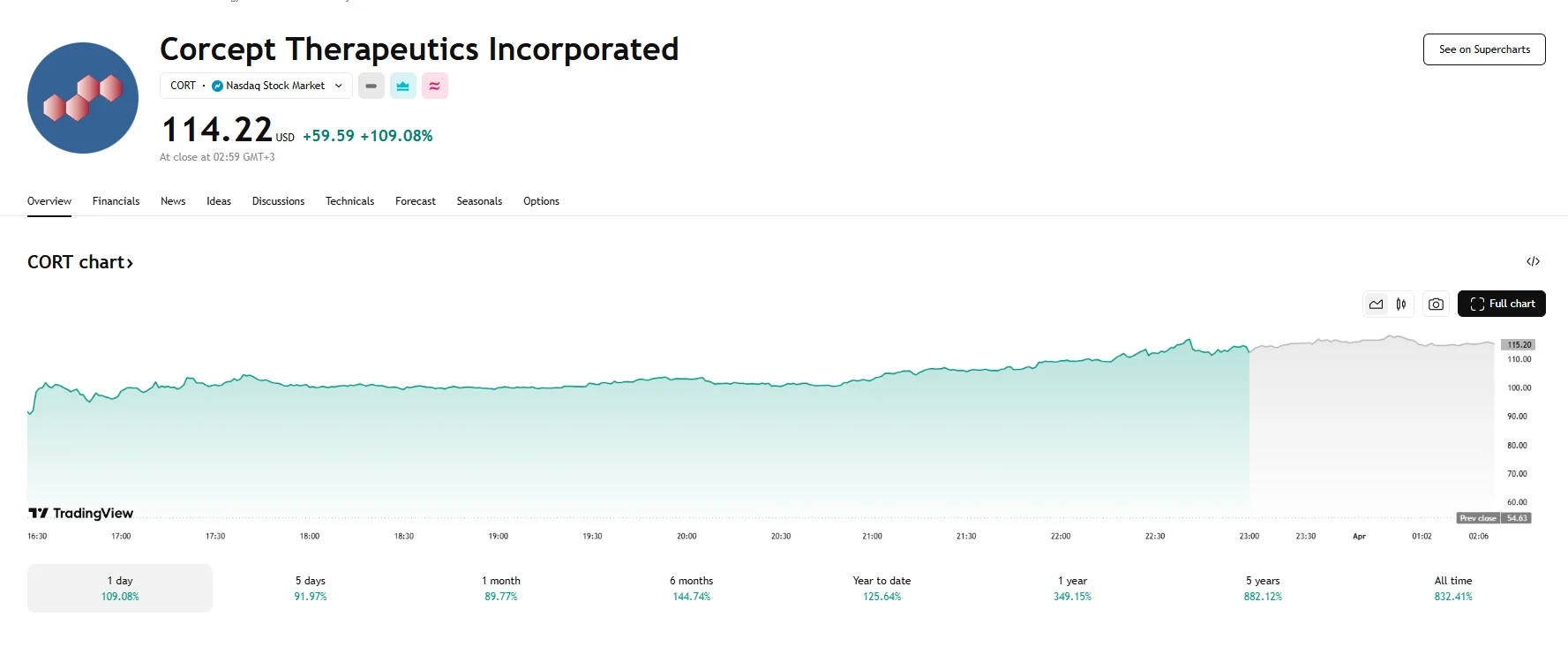
- Corcept Therapeutics’ stock soared 109% on Monday, closing at $114.22.
- The company’s pivotal Phase 3 ROSELLA study was a success, reducing ovarian cancer progression by 30% and stretching progression-free survival to 6.5 months.
- Corcept Therapeutics plans to file for regulatory authorization in the U.S. and Europe.
Corcept Therapeutics Shares Skyrocket to $114 Following Release of Positive Phase 3 Trial Data
Corcept Therapeutics (NASDAQ: CORT) achieved a monumental surge in stock value on Monday, propelled by the encouraging outcomes of its Phase 3 ROSELLA clinical trial. The company’s shares, initially trading at a modest level, skyrocketed, adding almost $60 to their value and reaching a striking $114.22. This impressive leap represented a 109% increase, and the stock maintained its elevated position on Tuesday, briefly touching $118.
The pivotal ROSELLA study examined the efficacy of relacorilant, an oral antiglucocorticoid medication, when combined with chemotherapy (nab-paclitaxel), in treating patients with platinum-resistant ovarian cancer. The study indicated that the combination therapy reduced the likelihood of disease advancement by 30%, resulting in a median progression-free survival of 6.5 months, surpassing the 5.5 months recorded for those receiving chemotherapy-only treatment. Moreover, the combined therapy demonstrated a notable improvement in median overall survival, reaching 16.0 months, which considerably exceeded the 11.5 months observed in the control group. According to the results, relacorilant did not induce a rise in adverse effects either.
The significance of these trials lies in their potential to transform the treatment paradigm for platinum-resistant ovarian cancer. Corcept’s relacorilant has shown remarkable promise in helping treat this particularly challenging condition. Crucially, patients could be enrolled without the need for biomarker selection. The treatment could, therefore, be viable for a variety of patient groups.
Corcept Therapeutics plans to leverage these positive results to seek regulatory approval in the United States and Europe. The company intends to submit a New Drug Application (NDA) to the U.S. Food and Drug Administration (FDA) in the third quarter of the year, followed by a Marketing Authorization Application (MAA) in Europe.
The successful trial also served to solidify Corcept Therapeutics’ position within the biotech industry. The company, known for its expertise in developing medications that modulate cortisol effects, has a proven track record, as evidenced by its existing product, Korlym, used in the treatment of Cushing’s syndrome. Relacorilant could lead to a major diversification of Corcept’s income generation and further strengthen its market position. Furthermore, the trial’s success has sparked speculation regarding Corcept’s potential as an acquisition target for larger pharmaceutical companies.





